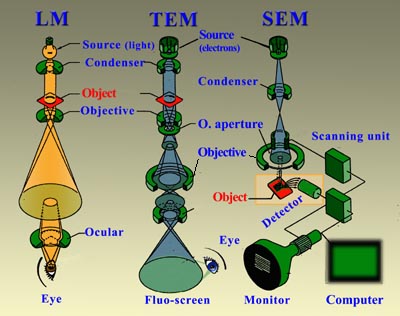
One of the things that you need to be aware of is the way in which organisms obtain their nutrition from. These terms may be handy.
- Photoautotraph - makes own food by using light energy. Photosynthesis is used by plants and some types of bacteria.
- Chemoautrotroph - make own food using the energy of simple chemicals. Bacteria found in deep ocean vents and hot springs are capable of this.
- Heterotroph - obtains food by ingesting complex materials from autotrophs or other heterotrophs.
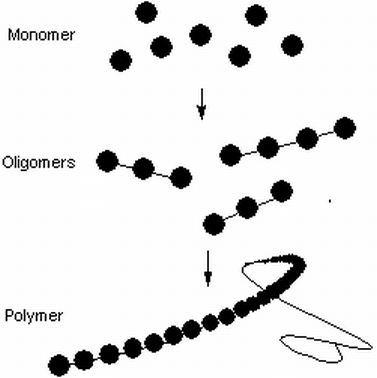
When it comes to nutrients, they can be arranged into a number of categories. Monomers are chemical groups that are made up of a single functional unit. These can be assembled into small chains of 2 or 3 units, called oligomers or long chains called polymers.
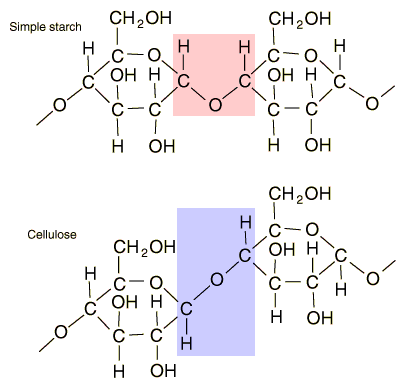
The first class of compounds are carbohydrates (CH2O - carbon + water). These provide energy when a monomer. The best known example is glucose. However, glucose monomers can be assembled into polymers for other uses:
Starch (plants) - longer term energy storage
Glycogen (animals) - longer term energy storage
Cellulose (plants) - material for cell walls, to provide rigidity and strength to plant cells.
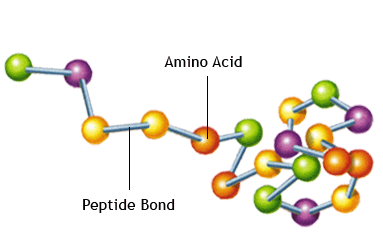
The second class of materials are proteins and amino acids. Amino acids are the build block for proteins. Proteins have many functions in a cell, but at this stage we will say that they are used for cell growth and repair.
Other classes of materials include fats and oils. These are used not only as in cell membranes but also as a long term energy storage because the amount of energy that they have is very high compared to other biological molecules.
Vitamins are a group of small molecules that are made by living things that assist many of the chemical reactions that take place in out cells.
Minerals include salts and more specifically dissolved ions. They can also assist with chemical reactions, but help regulate the water balance in cells and are involved with activities such as nerve signal transmission and muscle contraction.











.jpg)
.jpg)